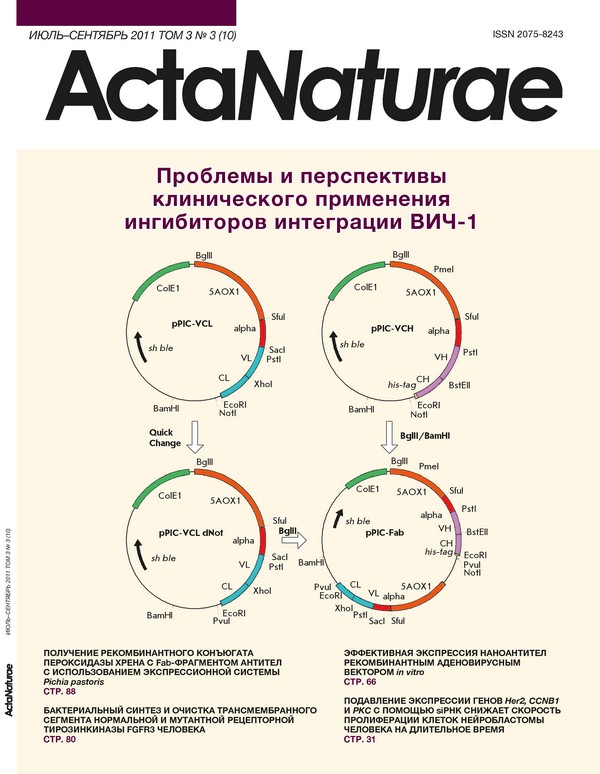
Recombinant Production of Horseradish Peroxidase Conjugates with Fab Antibodies in Pichia pastoris for Analytical Applications
- Authors: Koliasnikov OV1, Grigorenko VG2, Egorov AM2, Lange S3, Schmid RD3
-
Affiliations:
- Kolmogorov Advanced Education and Science Center, Lomonosov Moscow State University
- Lomonosov Moscow State University
- Institute of Technical Biochemistry, University of Stuttgart
- Issue: Vol 3, No 3 (2011)
- Pages: 85-92
- Section: Articles
- Submitted: 17.01.2020
- Published: 15.09.2011
- URL: https://actanaturae.ru/2075-8251/article/view/10697
- DOI: https://doi.org/10.32607/20758251-2011-3-3-85-92
- ID: 10697
Cite item