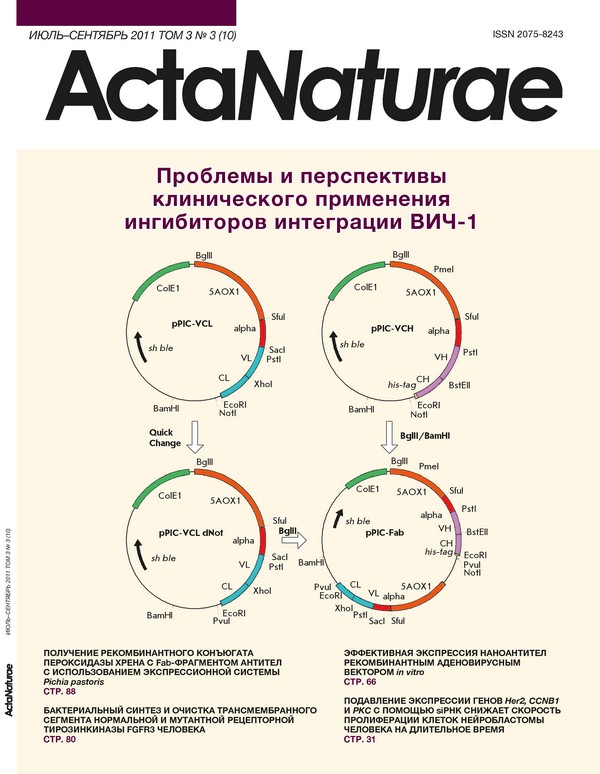
Recombinant Production of Horseradish Peroxidase Conjugates with Fab Antibodies in Pichia pastoris for Analytical Applications
- Authors: Koliasnikov OV1, Grigorenko VG2, Egorov AM2, Lange S3, Schmid RD3
-
Affiliations:
- Kolmogorov Advanced Education and Science Center, Lomonosov Moscow State University
- Lomonosov Moscow State University
- Institute of Technical Biochemistry, University of Stuttgart
- Issue: Vol 3, No 3 (2011)
- Pages: 85-92
- Section: Articles
- URL: https://actanaturae.ru/2075-8251/article/view/10697
- DOI: https://doi.org/10.32607/20758251-2011-3-3-85-92
- ID: 10697
Cite item
Abstract
Keywords
Full Text
Recombinant Production of Horseradish Peroxidase Conjugates with Fab Antibodies in Pichia pastoris for Analytical ApplicationsAbout the authors
O V Koliasnikov
Kolmogorov Advanced Education and Science Center, Lomonosov Moscow State UniversityDepartment of Chemistry
V G Grigorenko
Lomonosov Moscow State University
Email: vitaly@immunotek.ru
Department of Chemistry
A M Egorov
Lomonosov Moscow State UniversityDepartment of Chemistry
S Lange
Institute of Technical Biochemistry, University of Stuttgart
R D Schmid
Institute of Technical Biochemistry, University of Stuttgart
References
- Rau D., Kramer K., Hock B. // J. Immunoassay Immunochem. 2002. V. 23. № 2. Р. 129-143.
- Tachibana H., Takekoshi M., Cheng X.J., Nakata Y., Takeuchi T., Ihara S. // Clin. Diagn. Lab. Immunol. 2004. V. 11. №1. Р. 216-218.
- Mousli M., Turki I., Kharmachi H., Saadi M., Dellagi K. // J. Virol. Meth. 2007. V. 146. №1-2. Р. 246-256.
- Patel K.G., Ng P.P., Kuo C.C., Levy S., Levy R., Swartz J.R. // Biochem. Biophys. Res. Commun. 2009. V. 390. № 3. Р. 971-976.
- Joosten V., Roelofs M.S., van den Dries N., Goosen T., Verrips C.T., van den Hondel C.A., Lokman B.C. // J. Biotechnol. 2005. V. 120. № 4. Р 347-359.
- Grigorenko V., Andreeva I., Borchers T., Spener F., Egorov A. // Anal. Chem. 2001. V. 73. № 6. Р 1134-1139.
- Robin S., Petrov K., Dintinger T., Kujumdzieva A., Tellier C., Dion M. // Mol. Immunol. 2003. V. 39. № 12. Р 729-738.
- Cupit P.M., Whyte J.A., Porter A.J., Browne M.J., Holmes S.D., Harris W.J., Cunningham C. // Lett. Appl. Microbiol. 1999. V. 29. № 5. Р 273-277.
- Morawski B., Lin Z., Cirino P., Joo H., Bandara G., Arnold F.H. // Protein Eng. 2000. V. 13. № 5. Р 377-384.
- Pennell C.A., Eldin P. // Res. Immunol. 1998. V. 149. № 6. Р. 599-603.
- Fischer R., Drossard J., Emans N., Commandeur U., Hellwig S. // Biotechnol. Appl. Biochem. 1999. V. 30. Pt 2. Р 117-112.
- Freyre F.M., Vazquez J.E., Ayala M., Canaan-Haden L., Bell H., Rodriguez I., Gonzalez A., Cintado A., Gavilondo J.V. // J. Biotechnol. 2000. V. 76. № 2-3. Р 157-163.
- Takahashi K., Yuuki T., Takai T., Ra C., Okumura K., Yokota T., Okumura Y. // Biosci. Biotechnol. Biochem. 2000. V. 64. № 10. Р 2138-2144.
- Andrade E.V., Albuquerque F.C., Moraes L.M., Brigido M.M., Santos-Silva M.A. // J. Biochem. (Tokyo.) 2000. V. 128. № 6. Р. 891-895.
- Luo D., Geng M., Schultes B., Ma J., Xu D.Z., Hamza N., Qi W., Noujaim A.A., Madiyalakan R. // J. Biotechnol. 1998. V. 65. № 2-3. Р. 225-228.
- Powers D.B., Amersdorfer P., Poul M., Nielsen U.B., Shalaby M.R., Adams G.P., Weiner L.M., Marks J.D. // J. Immunol. Meth. 2001. V. 251. № 1-2. Р. 123-135.
- Hellwig S., Emde F., Raven N.P., Henke M., van der Logt P., Fischer R. // Biotechnol. Bioeng. 2001. V. 74. № 4. Р. 344-352.
- Sambrook J., Fritsch E.F., Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, N.Y.; Cold Spring Harbor Lab. Press, 1989.
- Lange S., Schmitt J., Schmid R.D. // J. Immunol. Meth. 2001. V. 255. Р. 103-114.
- Giersch T. // J. Agric. Food Chem. 1993. V. 41. № 6. Р .1006-1011.
- Braman J., Papworth C., Greener A. // Methods Mol. Biol. 1996. V. 57. Р 31-44.
- Grigorenko V., Chubar T., Kapeliuch Yu., Borchers T., Spener F., Egorov A. // Biocatal. Biotransform. 1999. V. 17. Р. 359-397.
- Ferrari R.P., Traversa S., de Gioia L., Fantucci P., Suriano G., Ghibaudi E.M. // J. Biol. Inorg. Chem. 1999. V. 4. № 1. Р 12-20.
- Kramer K., Hock B. // Food Agric. Immunol. 1996. V. 8. Р 97-109.
Supplementary files